(adding feline onset, duration, etc info.) |
|||
| (32 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| ⚫ | [[Image:Humu00ut.jpg|thumb|200px|left| Humulin U]]Ultralente insulin crystals are large and slowly absorbed because of their size, making this a [[long-acting]] insulin. At one time, this type of insulin was also produced in [[bovine]], [[porcine]] and mixed bovine-porcine forms. The two who most recently took their last bows are of [[r-DNA/GE/GM]] origin.<br><br> |
||
| ⚫ | [http://bnf.org/bnf/ British National Formulary] defines Ultralente-type [[insulins]] as: A sterile neutral (neutral used here refers to the pH, not to the fast-acting insulin known as neutral or R) suspension of bovine insulin or of human insulin in the form of a complex obtained by the addition of a suitable zinc salt; consists of rhombohedral crystals (10-40 microns). |
||
| ⚫ | Ultralente is not used very successfully in dogs, but has been widely used in treatment of cats. With an [[onset]] of 1-4 hours and a [[duration]] of 12-24 hours. Most cats require 2 ultralente shots a day. Ultralente [[absorption]] can be erratic, with about 20% not responding to the insulin--not even with twice-daily dosing<ref>[http://petdiabetes.wikia.com/images/d/d5/Providing_care_veterinary_diabetics.pdf Providing Care for Diabetic Veterinary Patients-International Journal of Pharmaceutical Compounding-2000-Page 3]</ref>.[[Image:Ultratardhm.png|thumb|200px|right|Novo Nordisk Ultratard HM-GE Human Ultralente insulin.]]<br><br> |
||
| ⚫ | Ultralente insulin crystals are large and slowly absorbed because of their size, making this a [[long-acting]] insulin. At one time, this type of insulin was also produced in [[bovine]], [[porcine]] and mixed bovine-porcine forms. The two who |
||
| + | |||
| + | Human ultralente insulin was not very reliable when used with many people, either. This is why it wasn't widely used enough to remain on either [[Eli Lilly]]'s or [[Novo Nordisk]]'s product list. There's great variability from patient to patient with this insulin<ref>[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11003253&dopt=Abstract Scandanavian Journal of Clinical/Laboratory Investigations-Use of Human Ultralente Limited By Great Intraindividual Variability]</ref>. Conclusion in this abstract is it has far too much day-to-day variability to be truly useful for many. |
||
| + | |||
| + | Some people [[absorption|absorb]] [[r-DNA]] ultralente at a faster than usual rate, so for them it would have only the [[duration]] of an [[:Category:intermediate-acting|intermediate acting]] insulin.<ref>[http://www.diabetes.org/uedocuments/rg06_insulin.pdf Diabetes Forecast-ADA, 2006-Page 2]</ref>. One injection of [[Bovine]] Ultralente insulin can last up to 40 hours when administered to a human--longer than [[Lantus]]; it is also [[peak|peakless]]<ref>[http://care.diabetesjournals.org/cgi/content/abstract/9/2/120?ijkey=3a37d4cefcd392dfa7f160decaaa29cab36bf37e&keytype2=tf_ipsecsha Use of Beef Ultralente for Basal Insulin Delivery-Diabetes Care-American Diabetes Association-1986]</ref><ref>[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=2635094&query_hl=3&itool=pubmed_docsum Comparison of the Safety and Effectiveness of Human and Bovine Long-acting Insulins-Diabetes Research-1989]</ref>. The human [[duration]] with [[Bovine]] ultralente is due, in part, to the [[Duration#Duration_By_Species|3 amino acid differences]] between bovine and human insulin. |
||
| + | |||
| + | Most humans not using an [[analog]] intermediate or long acting insulin for [[basal]] needs are or were using either [[NPH]] or [[Lente]] on an average of twice a day. |
||
| + | ==Specifications of ultralente== |
||
| + | |||
| ⚫ | British National Formulary<ref>[http://bnf.org/bnf/ British National Formulary-Ultralente Definition]</ref> defines Ultralente-type [[insulins]] as: A sterile neutral (neutral used here refers to the pH, not to the fast-acting insulin known as neutral or R) suspension of bovine insulin or of human insulin in the form of a complex obtained by the addition of a suitable zinc salt; consists of rhombohedral crystals (10-40 microns). |
||
| + | |||
| + | British Pharmacoepia (BP) and United States Pharmacoepia (USP)'s definitions of ultralente insulin:<ref>[http://www.inchem.org/documents/pims/pharm/insulin.htm British Pharmacoepia (BP) and United States Pharmacoepia (USP) Definitions of Ultralente Insulin]</ref> |
||
| + | |||
| + | Insulin zinc suspension (crystalline) BP |
||
| + | |||
| + | Sterile buffered suspension of bovine insulin to which zinc chloride is added. Crystalline form is insoluble in water. |
||
| + | Prepared from crystalline insulin containing not more than 23 units/mg. |
||
| + | White or almost colourless suspension. Particles are mainly crystalline. Majority of crystals having a maximum diameter greater than 10 m.pH 6.9 - 7.5 Iso-osmotic with blood. Preparation contains 40 and 80 units/ml. |
||
| + | |||
| + | (Note--the BP definition should actually read "mammalian", not "bovine". [[Novo Nordisk]]'s [[Ultratard]] and [[Eli Lilly]]'s [[Humulin Zn]] were both [[r-DNA/GE/GM]] insulins. Both were available until recently. Both were also U100 strength, so this strength should have been added above.) |
||
| + | |||
| + | U.S.P. - Sterile suspension of insulin contain 40, 80, 100 units/ml. Contains sodium acetate, sodium chloride and methyl hydroxybenzoate (Concentration same as for amorphous insulin) and zinc 120 - 250 ug. pH 7.2 - 7.5. |
||
| + | |||
| + | ==What Ultralente Is Not== |
||
| + | |||
| + | [[Image:Ultralentetap.gif|thumb|250px|Human time activity profile for r-DNA/GE/GM ultralente insulin.]]'''No''' Lente-type insulin regardless of species can contain any [[NPH]]/[[isophane]] insulin<ref>[http://web.archive.org/web/20060427044732/http://www.nfb.org/vodold/vsum9809.htm Combining Lente-type Insulins with Phenol-Preserved Insulins]</ref> '''or''' any [[R]]/[[Neutral]] insulin<ref>[http://www.endotext.org/diabetes/diabetes17/diabetes17.htm Lente Zinc Suspension Causes Loss Of R/Neutral Short-Acting Effect]</ref><ref>[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=retrieve&db=pubmed&list_uids=1863938&dopt=AbstractPlus Availability of Soluble (R/Neutral) Insulin in Mixed Preparations of Crystalline (Lente) & Ultralente GE Insulins-Clinical Therapeutics-1991]</ref>. |
||
| + | |||
| + | Both are chemically impossible: the phenol [[preservatives|preservative]] present in [[NPH]]/[[isophane]] alters the action of Lente-type insulins, creating a mixture with an approximate action of [[R]]/[[Neutral]]. |
||
| + | |||
| + | The zinc [[suspension]] of Lente-type insulin binds [[R]]/[[Neutral]], causing the [[short-acting]] insulin to slow, losing its short-acting effect. |
||
| + | |||
| + | ==Combining Lente Family Insulins== |
||
| + | |||
| + | {| |
||
| + | |-valign="top" |
||
| + | |style="height:1px;border:0;" colspan="3"| |
||
| + | |-valign="top" |
||
| + | |style="padding:5px;border:2px solid #ffb6c1;background-color:#ffe4e1;" width="50%"| |
||
| + | |||
| + | None of the Lente family of insulins ([[semilente]], [[Lente]], [[Ultralente]]) can be combined with<ref>[http://web.archive.org/web/20060427044732/http://www.nfb.org/vodold/vsum9809.htm Phenol Preservatives & Lente-type Insulins--A Bad Combination]</ref> [[NPH]]/[[isophane]] insulins. The phenol [[preservatives]] present in [[NPH]]-type insulins alters the [[Lente]]-types to the point where they become a close approximation of [[R]]/[[neutral]], with regard to action<ref>[http://web.archive.org/web/20070820011317/http://www.rxed.org/rxtech/ce/tech-insulin.htm RxEd.org-Insulin Therapy-Mixing Precautions]</ref>. |
||
| + | |||
| + | Keeping the phenol [[preservatives]] in mind, all [[protamine]]-[[Suspension|suspended]] insulin mixes would be "off limits" regarding same syringe mixing with any [[Lente]]-type insulins<ref>[http://web.archive.org/web/20070820011317/http://www.rxed.org/rxtech/ce/tech-insulin.htm RxEd.org-Insulin Therapy-Mixing Precautions]</ref>. |
||
| + | |} |
||
| + | |||
| + | Insulin manufacturers<ref>[http://www.endotext.org/diabetes/diabetes17/diabetes17.htm Insulin Producers vs Doctors Re:Combining R/Neutral & Lente-type Insulins]</ref> indicate that [[R]]/[[neutral]] and [[semilente]], [[Lente]], [[ultralente]] insulins are able to be combined in the same syringe, but only just before injection. In pre-filled syringes, the zinc [[suspension]] of the [[Lente]]-type insulins binds the [[R]]/[[neutral]], causing it to lose its [[short-acting]] effect. Various studies have documented this, and some doctors advise against using [[R]]/[[neutral]] in the same syringe with the [[Lente]] family of insulins<ref>[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=retrieve&db=pubmed&list_uids=1863938&dopt=AbstractPlus Availability of Soluble (R/Neutral) Insulin in Mixed Preparations of Crystalline (Lente) & Ultralente GE Insulins-Clinical Therapeutics-1991]</ref><ref>[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?itool=abstractplus&db=pubmed&cmd=Retrieve&dopt=abstractplus&list_uids=3304896 Absorption Kinetics & Action Profiles-Single Subcutaneous Administration of Human Soluble (R/Neutral) & Lente Insulin-Diabetes Care-1987]</ref><ref>[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=retrieve&db=pubmed&list_uids=3545725&dopt=AbstractPlus Delayed Onset of Action of Soluble (R/Neutral) Insulin After Premixing With Lente Insulin Diabetes Research & Clinical Practice-1983]</ref><ref>[http://web.archive.org/web/20070820011317/http://www.rxed.org/rxtech/ce/tech-insulin.htm RxEd.org-Insulin Therapy-Mixing Precautions]</ref>. |
||
| ⚫ | Ultralente is not used very successfully in dogs, but has been widely used in treatment of cats. With an [[onset]] of 1-4 hours and a [[duration]] of 12-24 hours. Most cats require 2 ultralente shots a day. Ultralente [[absorption]] can be erratic, with about 20% not responding to the insulin--not even with twice-daily [http:// |
||
Trade names: |
Trade names: |
||
| + | {| align=center width="400" border="1" style="background:powderblue;" | |
||
| ⚫ | |||
| + | |- |
||
| + | ! colspan=2 align="center" style="background:powderblue;"| |
||
| + | '''Ultralente Insulins''' |
||
| + | |- |
||
| + | ! colspan=2 align="center" style="background:lightcyan;"| |
||
| + | [[:Category:Long-acting|Long acting]] |
||
| + | |- |
||
| + | |||
| + | ! width="200"align="center" style="background:aliceblue;"| |
||
| + | [[R-DNA]] |
||
| ⚫ | |||
| + | |- |
||
| + | ! width="200"align="center" style="background:aliceblue;"| |
||
| + | [[R-DNA]] |
||
| ⚫ | |||
| + | |} |
||
| + | |||
| + | ==Further Reading== |
||
| + | |||
| + | [[Image:Wikicat3.jpg|100px]] |
||
| + | *[[:Category:Humulin U cases|Wiki cases--Feline Ultralente users]] |
||
| ⚫ | |||
| + | *[http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=Abstract&list_uids=8141485&query_hl=1&itool=pubmed_DocSum Comparison of 2 Ultralente Insulin Preparations with Protamine Zinc Insulin in Normal Cats-American Journal of Veterinary Research-1994] |
||
| + | ==References== |
||
| + | <references/> |
||
| Line 17: | Line 83: | ||
[[Category:Insulins]][[Category:long-acting]] |
[[Category:Insulins]][[Category:long-acting]] |
||
[[Category:r-DNA/GE/GM]] |
[[Category:r-DNA/GE/GM]] |
||
| + | [[Category:Terms]][[Category:Lente]] |
||
Latest revision as of 01:36, 18 August 2009

Humulin U
Ultralente insulin crystals are large and slowly absorbed because of their size, making this a long-acting insulin. At one time, this type of insulin was also produced in bovine, porcine and mixed bovine-porcine forms. The two who most recently took their last bows are of r-DNA/GE/GM origin.
Ultralente is not used very successfully in dogs, but has been widely used in treatment of cats. With an onset of 1-4 hours and a duration of 12-24 hours. Most cats require 2 ultralente shots a day. Ultralente absorption can be erratic, with about 20% not responding to the insulin--not even with twice-daily dosing[1].

Novo Nordisk Ultratard HM-GE Human Ultralente insulin.
Human ultralente insulin was not very reliable when used with many people, either. This is why it wasn't widely used enough to remain on either Eli Lilly's or Novo Nordisk's product list. There's great variability from patient to patient with this insulin[2]. Conclusion in this abstract is it has far too much day-to-day variability to be truly useful for many.
Some people absorb r-DNA ultralente at a faster than usual rate, so for them it would have only the duration of an intermediate acting insulin.[3]. One injection of Bovine Ultralente insulin can last up to 40 hours when administered to a human--longer than Lantus; it is also peakless[4][5]. The human duration with Bovine ultralente is due, in part, to the 3 amino acid differences between bovine and human insulin.
Most humans not using an analog intermediate or long acting insulin for basal needs are or were using either NPH or Lente on an average of twice a day.
Specifications of ultralente
British National Formulary[6] defines Ultralente-type insulins as: A sterile neutral (neutral used here refers to the pH, not to the fast-acting insulin known as neutral or R) suspension of bovine insulin or of human insulin in the form of a complex obtained by the addition of a suitable zinc salt; consists of rhombohedral crystals (10-40 microns).
British Pharmacoepia (BP) and United States Pharmacoepia (USP)'s definitions of ultralente insulin:[7]
Insulin zinc suspension (crystalline) BP
Sterile buffered suspension of bovine insulin to which zinc chloride is added. Crystalline form is insoluble in water. Prepared from crystalline insulin containing not more than 23 units/mg. White or almost colourless suspension. Particles are mainly crystalline. Majority of crystals having a maximum diameter greater than 10 m.pH 6.9 - 7.5 Iso-osmotic with blood. Preparation contains 40 and 80 units/ml.
(Note--the BP definition should actually read "mammalian", not "bovine". Novo Nordisk's Ultratard and Eli Lilly's Humulin Zn were both r-DNA/GE/GM insulins. Both were available until recently. Both were also U100 strength, so this strength should have been added above.)
U.S.P. - Sterile suspension of insulin contain 40, 80, 100 units/ml. Contains sodium acetate, sodium chloride and methyl hydroxybenzoate (Concentration same as for amorphous insulin) and zinc 120 - 250 ug. pH 7.2 - 7.5.
What Ultralente Is Not
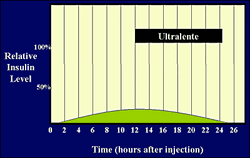
Human time activity profile for r-DNA/GE/GM ultralente insulin.
No Lente-type insulin regardless of species can contain any NPH/isophane insulin[8] or any R/Neutral insulin[9][10].
Both are chemically impossible: the phenol preservative present in NPH/isophane alters the action of Lente-type insulins, creating a mixture with an approximate action of R/Neutral.
The zinc suspension of Lente-type insulin binds R/Neutral, causing the short-acting insulin to slow, losing its short-acting effect.
Combining Lente Family Insulins
|
None of the Lente family of insulins (semilente, Lente, Ultralente) can be combined with[11] NPH/isophane insulins. The phenol preservatives present in NPH-type insulins alters the Lente-types to the point where they become a close approximation of R/neutral, with regard to action[12]. Keeping the phenol preservatives in mind, all protamine-suspended insulin mixes would be "off limits" regarding same syringe mixing with any Lente-type insulins[13]. | ||
Insulin manufacturers[14] indicate that R/neutral and semilente, Lente, ultralente insulins are able to be combined in the same syringe, but only just before injection. In pre-filled syringes, the zinc suspension of the Lente-type insulins binds the R/neutral, causing it to lose its short-acting effect. Various studies have documented this, and some doctors advise against using R/neutral in the same syringe with the Lente family of insulins[15][16][17][18].
Trade names:
|
Ultralente Insulins | |
|---|---|
| Humulin U, Humulin Zn (No longer produced.) | |
| Novolin U, Ultratard (No longer produced.) | |
Further Reading
References
- ↑ Providing Care for Diabetic Veterinary Patients-International Journal of Pharmaceutical Compounding-2000-Page 3
- ↑ Scandanavian Journal of Clinical/Laboratory Investigations-Use of Human Ultralente Limited By Great Intraindividual Variability
- ↑ Diabetes Forecast-ADA, 2006-Page 2
- ↑ Use of Beef Ultralente for Basal Insulin Delivery-Diabetes Care-American Diabetes Association-1986
- ↑ Comparison of the Safety and Effectiveness of Human and Bovine Long-acting Insulins-Diabetes Research-1989
- ↑ British National Formulary-Ultralente Definition
- ↑ British Pharmacoepia (BP) and United States Pharmacoepia (USP) Definitions of Ultralente Insulin
- ↑ Combining Lente-type Insulins with Phenol-Preserved Insulins
- ↑ Lente Zinc Suspension Causes Loss Of R/Neutral Short-Acting Effect
- ↑ Availability of Soluble (R/Neutral) Insulin in Mixed Preparations of Crystalline (Lente) & Ultralente GE Insulins-Clinical Therapeutics-1991
- ↑ Phenol Preservatives & Lente-type Insulins--A Bad Combination
- ↑ RxEd.org-Insulin Therapy-Mixing Precautions
- ↑ RxEd.org-Insulin Therapy-Mixing Precautions
- ↑ Insulin Producers vs Doctors Re:Combining R/Neutral & Lente-type Insulins
- ↑ Availability of Soluble (R/Neutral) Insulin in Mixed Preparations of Crystalline (Lente) & Ultralente GE Insulins-Clinical Therapeutics-1991
- ↑ Absorption Kinetics & Action Profiles-Single Subcutaneous Administration of Human Soluble (R/Neutral) & Lente Insulin-Diabetes Care-1987
- ↑ Delayed Onset of Action of Soluble (R/Neutral) Insulin After Premixing With Lente Insulin Diabetes Research & Clinical Practice-1983
- ↑ RxEd.org-Insulin Therapy-Mixing Precautions